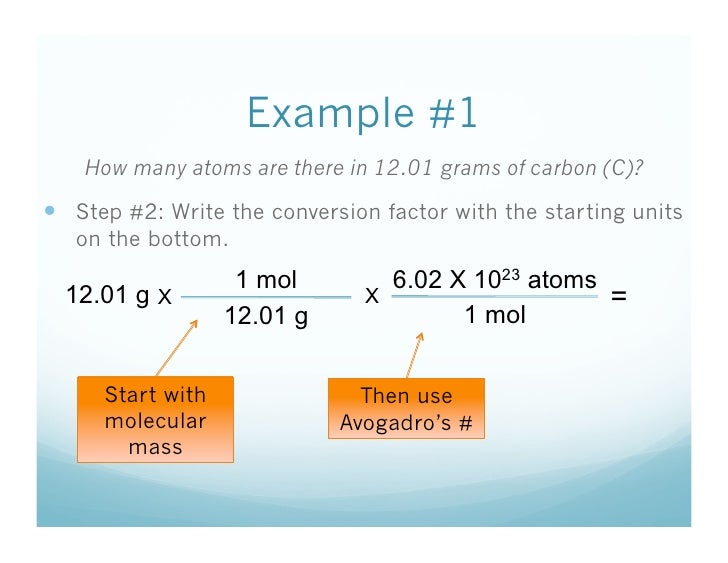
Avogadro's number is one of the most important constants used in chemistry. It is the number of particles in a single mole of a material, based on the number of atoms in exactly 12 grams of the isotope carbon-12. Although this number is a constant, it's experimentally determined, so we use an approximate value of 6.022 x 1023. So, you know how many atoms are in a mole. Here's how to use the information to determine the mass of a single atom.
Cara download app world blackberry torchlight. Posts about cara download blackberry app world written by Ferry Tanu Putra. Trik dan Tips Blackberry. Informasi, review, trik dan tips blackberry. Jika ingin download BlackBerry App World dari BlackBerry anda langsung. BlackBerry Torch 2: Review, Foto dan Spesifikasi; BlackBerry Bold Dakota: Review, Foto dan Spesifikasi.
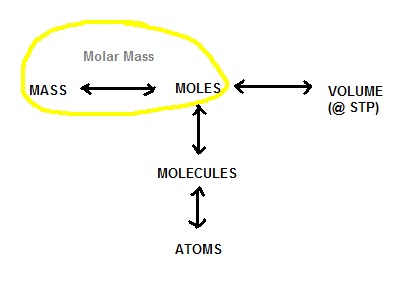
One mol of atoms of carbon weight indeed 12 grams. But one mol of atoms of Hidrogen weight one gramm. And one mol of molecules of Hidrogens weight two gramms because each molecule bears two atoms H2. That's why chemist say the atomic mass (or weight) of carbon is 12 (because one mol of atoms of carbon weights 12 grams). Atoms To Mass In Grams Converter Kilograms. We couldn't find a conversion between atoms and grams [incompatible types] Quickly convert atoms into grams-force (atoms to grams) using the online calculator for. 0 Comments Leave a Reply. Write something about yourself. No need to be fancy, just an overview. It is not possible to convert the given substance in grams to atoms. We assume you are converting between grams C6H12O6 and mole. You can view more details on each measurement unit: or This compound is also known as. The SI base unit for is the mole. 1 grams C6H12O6 is equal to 0.072617 mole.
Avogadro's Number Example Problem: Mass of a Single Atom
Question: Calculate the mass in grams of a single carbon (C) atom.
Solution
To calculate the mass of a single atom, first look up the atomic mass of carbon from the periodic table.
This number, 12.01, is the mass in grams of one mole of carbon. One mole of carbon is 6.022 x 1023 atoms of carbon (Avogadro's number). This relation is then used to 'convert' a carbon atom to grams by the ratio:
mass of 1 atom / 1 atom = mass of a mole of atoms / 6.022 x 1023 atoms
Plug in the atomic mass of carbon to solve for the mass of 1 atom:
mass of 1 atom = mass of a mole of atoms / 6.022 x 1023
mass of 1 C atom = 12.01 g / 6.022 x 1023 C atoms
mass of 1 C atom = 1.994 x 10-23 g
Answer
The mass of a single carbon atom is 1.994 x 10-23 g.
Applying the Formula to Solve for Other Atoms and Molecules
Although the problem was worked using carbon (the element upon which Avogadro's number is based), you can use the same method to solve for the mass of an atom or molecule. If you're finding the mass of an atom of a different element, just use that element's atomic mass.
If you want to use the relation to solve for the mass of a single molecule, there's an extra step. You need to add up the masses of all of the atoms in that one molecule and use them instead.
Let's say, for example, you want to know the mass of a single atom of water. From the formula (H2O), you know there are two hydrogen atoms and one oxygen atom. You use the periodic table to look up the mass of each atom (H is 1.01 and O is 16.00). Forming a water molecule gives you a mass of:
ProLingo English Finnish Dictionary 1.4.7 Language software developed by ProLingo Software. The license of this language software is shareware$, the price is 27.95, you can free download and get a free trial before you buy a registration or license. Keep your photos safe in the cloud with the best online photo storage for 2019 Stay private and protected with the best Firefox security extensions Clean out junk files in Windows 7, 8.1, and 10. This is English - Finnish and Finnish - English Dictionay (suomi-englanti-suomi sanakirja), containing 47000 translation articles. The Dictionary is OFFLINE and does not need the internet connection. Database size is more than 11MB. It will be downloaded when the application is run first time. We recommend you to use Wi-Fi connection. Finnish translation. Windows 7 Ready! Slang words Included. Audio pronunciation in 5 voices. Contains most of available words and is great solution same as for novice and experienced skill levels.
1.01 + 1.01 + 16.00 = 18.02 grams per mole of water
and you solve with:
mass of 1 molecule = mass of one mole of molecules / 6.022 x 1023
mass of 1 water molecule = 18.02 grams per mole / 6.022 x 1023 molecules per mole
mass of 1 water molecule = 2.992 x 10-23 grams
››Convert gram-force to mole
Please enable Javascriptto use the unit converter
Error: We couldn't find a conversion between grams and moles[incompatible types]
To complete this calculation, you need to know what substanceyou are trying to convert. The reason is that the molar massof the substance affects the conversion.
Molar mass has units of grams per mole (g/mol). It is calculatedby measuring the molecular weight of the chemical compound, whichtells us how many grams are in one mole of that substance. Theformula weight is simply the weight in atomic mass units of allthe atoms in a given formula.
We can compute the molar mass for you. All you have to do isenter the chemical formula, or the name of the chemical compound.
Here are some other examples:
grams SiS2 to moles
grams InCl to moles
grams TmCl2.7H2O to moles
grams CdCrO4 to moles
grams Rubidium to moles
grams Phosphorus Decaoxide to moles
grams Cadmium Nitride to moles
grams Magnesium Sulfide to moles
grams Sodium Periodate to moles
Were you trying to convert force units or amount of substance units?
Having trouble with a unit conversion?
Try doing asearch,or posting to theforum.
You may also be looking for adate difference ormolecular weight.
You can alsocontact us directlyif you find any missing units or errors.
››Want other units?
››Definition: Mole
The mole is the amount of substance of a system which contains as many elementary entities as there are atoms in 0.012 kilogram of carbon 12; its symbol is 'mol.'
Grams To Atoms
››Metric conversions and more
How Do You Convert Atoms To Mass In Grams
ConvertUnits.com provides an onlineconversion calculator for all types of measurement units.You can find metric conversion tables for SI units, as wellas English units, currency, and other data. Type in unitsymbols, abbreviations, or full names for units of length,area, mass, pressure, and other types. Examples include mm,inch, 100 kg, US fluid ounce, 6'3', 10 stone 4, cubic cm,metres squared, grams, moles, feet per second, and many more!